Fully integrated commercial scale facilities
Biosimilar Manufacturing Capabilities
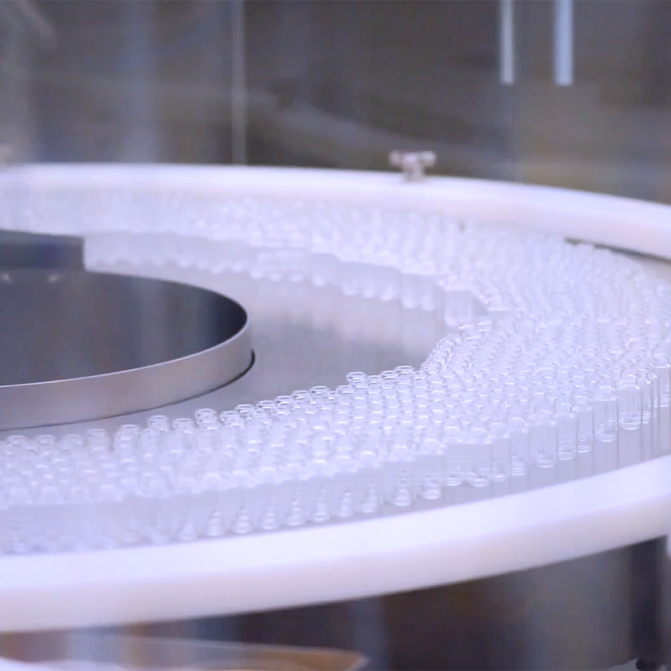
Our biosimilars manufacturing centre in Hyderabad, India, is a fully integrated commercial scale facility. It has end-to-end capabilities in producing a full range of products from bulk drug substance (DS) to fill-finish and packaged drug products (DP).

Our dedicated mammalian and microbial production suites are built for 24:7 operation, with manufacturing capable of producing:

600kg
of monoclonal antibodies (mABs) per year

80
mammalian cell culture batches per year

150
microbial cell culture batches per year

The goal of our biosimilar manufacturing facility is to produce medicines of the highest quality and affordability.
CuraTeQ has strategically invested in its commercial scale biosimilar manufacturing facilities with world class equipment and infrastructure, which is globally compliant with various international regulatory norms.
It is a fully integrated modular facility with selectively automated production processes and systems to minimise human intervention. Our dedicated team of expert process engineers, production engineers, and operators are instrumental to the efficient and reliable operation of the facility.
Key features of our biosimilar manufacturing facilities

140,000 sq. ft. multi product manufacturing facility

Designed for optimal man and material movement to mitigate cross contamination

Conceptualised with Quality-by-Design (QbD) principles

Designed for Clean-In-Place (CIP) and Sterilisation-In-Place (SIP) operations

Segregated areas for mammalian and microbial bulk Drug Substance (DS) manufacturing

Automated buffer distribution and handling capabilities

Segregated fill finish lines for Vials and Pre-filled syringes/cartridges

Systems designed as per 21 CFR Part 11, and GAMP 5 ISPE guidelines

Centrally located warehouse for ease of servicing various manufacturing blocks

Continuous monitoring through Building Management Systems (BMS)
Biosimilar manufacturing facility deep dive
Select an area:

Engineering and Support Utilities
Our manufacturing facility is equipped with dedicated HVAC units with terminal HEPA filters in process areas, which provide clean filtered air to avoid cross contamination. Our manufacturing plant also houses various critical utilities in a dedicated “Utility Block” including:
- Water for Injection (WFI) production facility & Pure steam generators
- Gas distribution systems (Liquid Oxygen & Nitrogen)
- Chillers (400 TR capacity) and Air dryers (122 CFM capacity)
- Boilers to generate plant steam (6 Ton cumulative capacity)
- UPS (120 KVA) and Power Backup/DG (1000 KVA, 1500 KVA)