Driving biosimilar development from Gene to GMP
Biosimilar Research and Development Capabilities
Developing high quality biosimilars requires a strong research and development footprint and expert scientific resources.

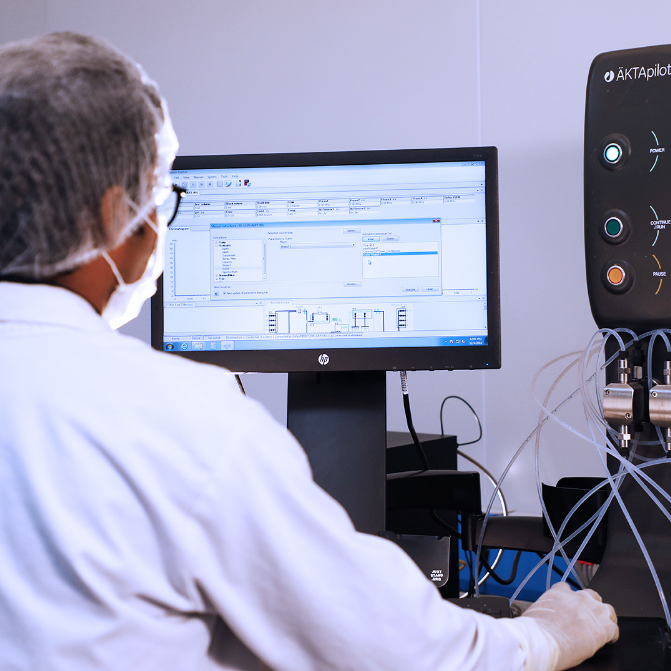
The Biologics Research and Development Centre at CuraTeQ is a fully equipped world class facility spanning 40,000 sq. ft. The facility has dedicated laboratories to support all biosimilar development activities encompassing:
- Cell line development and fermentation sciences
- Process development
- Process characterisation
- Analytical product characterisation
- Formulation development
- Cell based functional assays development
- Clinical immunology
- Scale down model development and up-scaling
Key features of our biosimilar research and development capabilities

Staffed with 100+ highly qualified scientists experienced in novel biologics and biosimilars development

PhD to Masters ratio at 1:3

Extensive product characterisation experience using cutting edge analytical and bioanalytical tools

Good handle of process science and molecular tuning approaches ensuring process consistency and scalability

Strong regulatory focus

A strong network of regulatory and clinical sciences consultants

Integrated and centralised Laboratory Information Management System (LIMS)

Dedicated warehouse, cold rooms, and stability chambers
Since its inception, the Biologics Research and Development Centre at CuraTeQ has been instrumental in developing a robust and strategic pipeline of over 14 biosimilar products.